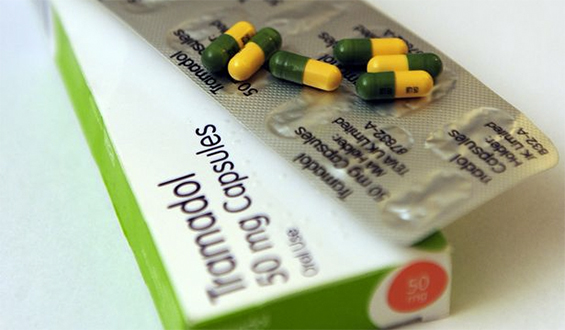
The US Food and Drug Administration is investigating the use of a pain pill called Tramadol by young people under the age of 17 due to the side-effect of dangerously slow breathing. Although the drug is not approved for children, doctors often prescribe it to them anyway. Maggie Fox of NBC News reports this kind of use is legal, but is not recommended. The FDA has issued a warning against the use of Tramadol among children and teenagers.
Other symptoms that can present include difficult or noisy breathing, confusion, or unusual sleepiness. If any of these occur, parents should stop use of the drug and get to an emergency room or call 911 immediately.
“This risk may be increased in children treated with tramadol for pain after surgery to remove their tonsils and/or adenoids,” the FDA said in a statement.
This pain pill is an opioid which the body converts to a morphine-like drug upon ingestion. These types of drugs can slow breathing and can result in death. A serious condition can develop if the patient is an “ultra-metabolizer.” This person’s liver converts the drug into its active form more quickly and completely than others, and the result is a type of overdose.
“Untreated pain can potentially result in long-term physical and psychological consequences. There are other pain medicines available that do not have this side effect of slowed or difficult breathing associated with tramadol and are FDA-approved for use in children,”the FDA announced.
Tramadol is also sold under the names Ultram, Ultram ER, Conzip, Ultracet, FusePaq Synapryn, Rybix ODT, Ryzolt, Theratramadol-60, Theratramadol-90, and as a generic, according to the US National Library of Medicine. The drug comes in liquid, capsule, and tablet form and is known as an opioid analgesic.
Although this kind of reaction is rare, parents and caregivers need to address any questions or concerns about tramadol or any other prescribed medications with their child’s health care professionals. The FDA investigation of tramadol is continuing, says Alex Lindley writing for Daily RX.
“Recently, a 5-year-old child in France experienced severely slowed and difficult breathing requiring emergency intervention and hospitalization after taking a single prescribed dose of tramadol oral solution for pain relief following surgery to remove his tonsils and adenoids,” the FDA said.
It was found that the child was an ultra-metabolizer and had high levels of O-desmethltramadol.
Another pain drug was recently approved by the FDA for children. Oxycontin may be administered if the FDA guidelines for how to prescribe the drug to children and teens are followed. Dr. Sharon Hertz, director of new anesthesia, analgesia and addiction products for the FDA, said studies by Purdue Pharma of Stamford, Connecticut, which manufactures Oxycontin, have supported the use of the drug for patients aged 11 to 16.
Oxycontin is a pain reliever that is a long-release version of oxycodone which acts on the brain like heroin and is to be used for only the most severe and chronic pain, says M. Alex Johnson for NBC News. He continued by stating that oxycontin is not intended to be the first choice of opioid drugs to be used in pediatric patients, but research has shown that changing from another opioid drug to oxycontin is not dangerous if used as directed.